Introduction to corrosion process
OBJECTIVE/SCOPE
To give young architects and engineers a basic understanding of the corrosion process and the practical means of protecting structural steelwork.
SUMMARY
This lecture presents the theory of corrosion in a very simple way. The galvanic series and galvanic corrosion are covered briefly. The question of why structural steel requires protection against corrosion is discussed and the fundamental considerations relating to protection are described, e.g. establishing the environment, the choices of protective coatings, surface preparation options, and other considerations, e.g. chemical cleanliness.
Related content
1. INTRODUCTION
The more common metals exist in nature as metallic compounds. The principal compounds of ores are oxides and sulphides.
The extraction process is:
Compound → Metal
Metals spontaneously react with any liquid or gaseous environment in which they are placed and a corrosion product is produced which is very similar to the original ore from which the metal was obtained. Thus:
Iron ore = Iron oxide
Rust = Iron oxide plus chemically bonded water
Corrosion processes are chemical reactions taking place at the surface of the metal. They obey well established chemical laws, which is fine if you know them! Most of us do not need to know them because we are not dealing with corrosion problems daily. The purpose of this lecture is to describe the main types of corrosion met in ordinary buildings, structures, plants, factories, etc.
Corrosion products may act as a barrier between the metal and its surroundings, effectively slowing down the corrosion rate. This phenomenon is frequently observed when metals corrode in air, a process known as 'dry corrosion.' It cannot be expected to happen when the corrosion products are soluble and the corrosion is taking place in an aqueous environment, i.e. 'wet corrosion.' For example, in a dry environment:
Zinc + Oxygen → Zinc oxide + Water + Oxygen
Nothing much happens!
But add acid condensate (as frequently occurs in industrial environments) and:
Zinc oxide + Sulphuric acid → Zinc sulphate + water → Eases away, exposing zinc
1.1 Dry corrosion
At room temperature, most metals carry a very thin oxide layer as a result of the metal's reaction with oxygen in the atmosphere. Metals subjected to heating may well carry a heavier layer or the layer may detach. For example, steel which has been hot rolled has a complex oxide layer which is physically unstable but still has a protective value provided the steel remains in air and as long as the layer remains a continuous layer.
Zinc in air carries a fairly protective film of zinc oxide, which increases in thickness very slowly. Aluminium carries a thin, highly protective oxide layer.
Dry corrosion may seem unlikely. However, it is worth remembering some corrosion takes place even under completely dry conditions. It needs removing before applying any form of protective coating.
1.2 Wet corrosion
Wet corrosion takes place in wet environments, i.e. where relative humidity exceeds 60%. These environments can be neutral, acid, or alkaline.
There may be uniform destruction of the metal, e.g. oxidation or, localised destruction, i.e. pitting and stress corrosion. The destruction can be concentrated at areas adjacent to a more noble metal or at points where the oxygen supply is limited.
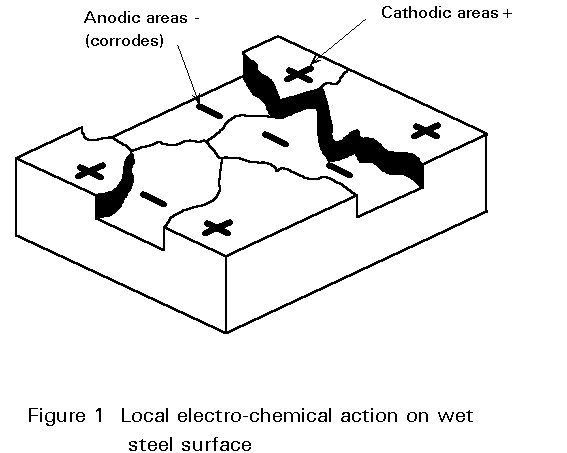
Wet corrosion is electro-chemical. When a metal is immersed in a conductive liquid (sulphur compounds in water in an industrial atmosphere or sodium chloride in water in a marine environment) some areas have a different electrical resistance from the rest of the surface (see Figure 1). A 'positive' electric current flows from the negative (-) anode to the positive (+) cathode areas and this leads to the dissolving or 'corroding' of the anode (see Figure 2). In other words, a 'corrosion cell' is, broadly speaking, the same as a car battery.
In corrosion prevention literature the terms 'galvanic' and the 'galvanic series' are frequently used.
Galvanic corrosion is the destruction of the less noble of a pair of metals joined together, e.g. in a sea water zinc is less noble than mild steel and the zinc wastes away rapidly.
The galvanic series is a list of metals arranged in order of their corrosion potential with the most easily corroded at the top and the least active at the bottom. The simplified listing which follows shows why aluminium and zinc, and magnesium more recently, are used as coatings to protect carbon steel and why stainless steel could replace it in certain circumstances.
Magnesium
Aluminium
Zinc
Iron & cast iron
Carbon Steel
Lead
Copper
Nickel
Silver
Austenitic stainless steel (316L)
Gold
Platinum
The most active metals, e.g. zinc and aluminium plus magnesium, are described as having negative electrical potentials. They may be referred to as anodic. The least active, e.g. gold and platinum, are referred to as noble or, cathodic.
When dissimilar metals are connected in the presence of an electrolyte, the more noble (cathodic) one tends to be protected while the more active (anodic or negative) one corrodes rapidly.
As the potential difference between two dissimilar metals increases so does the possibility for galvanic corrosion.
To sum up, the corrosion of metals is simply a reversion of their extraction process. 90% of marine and industrial corrosion is electro-chemical and the reaction approximates to that which takes place in a car battery.
1.3 Why protect steel?
Basically, steel is an alloy iron and carbon with other elements being added depending upon the processing method and the final performance required. Structural steels contain generally between 0.12% to 0.24% carbon. It was noted above that iron ore is iron oxide and rust is iron oxide plus chemically bonded water. Steel is man-made and unstable. It combines readily with oxygen and water, producing an iron oxide not unlike the original iron ore prior to refining.
Electro-chemical corrosion can be highly concentrated at certain points. If this occurs, a high rate of destruction at points representing no more than 1% of the total surface area can destroy the usefulness of a steel component. There are a number of reasons why local corrosion can be so concentrated:
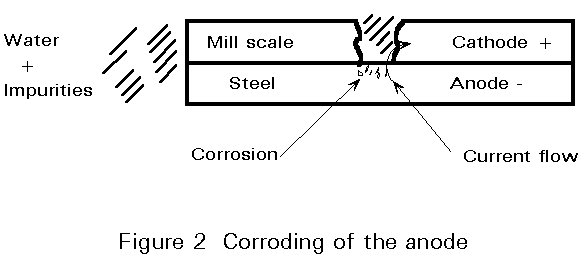
- The first reason is related to the presence of mill scale (see Figure 2). Much of the structural steel which is used is hot rolled. Hot steel is formed into structural sections by passing it through compressing rollers. In some parts of the process, water is poured upon the forming steel. Both of these operations cause an oxide layer to be built up on the steel's surface.
This oxide layer on hot rolled carbon steel, mill scale, is physically unstable. First, it is a separate entity from the steel in much the same way as is a coat of paint.
- Second, the mill scale is not a continuous layer and does not represent a protective barrier.
- Third, the mill scale is cathodic and the steel anodic. If there are a few breaks in the scale layer, a little condensation with dissolved impurities to act as the electrolyte and perhaps some dissolved oxygen and then a corrosion cell is formed, in which the steel dissolves (corrodes) away.
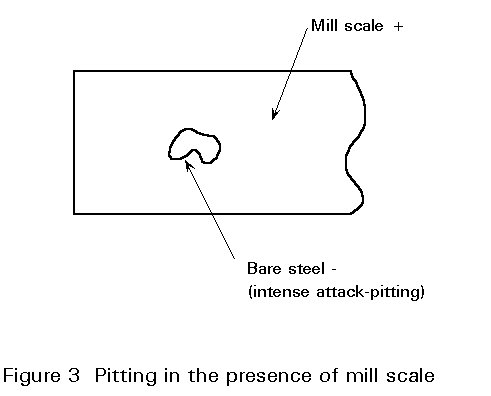
- Fourth, small bare areas of steel in large patches of intact mill scale, i.e. large cathodic areas, give rise to intense attack and severe pitting of the steel (see Figure 3).
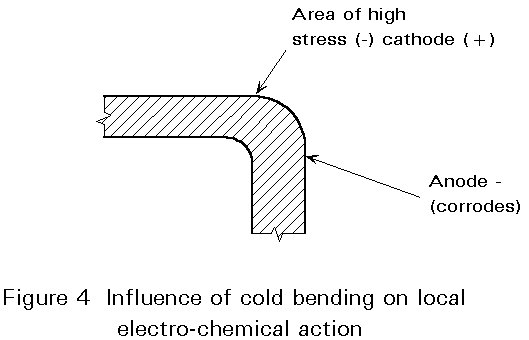
- Fifth, cold bending, welding, etc. can produce highly stressed areas with adjacent anodic (-) and cathodic (+) patches (see Figure 4).
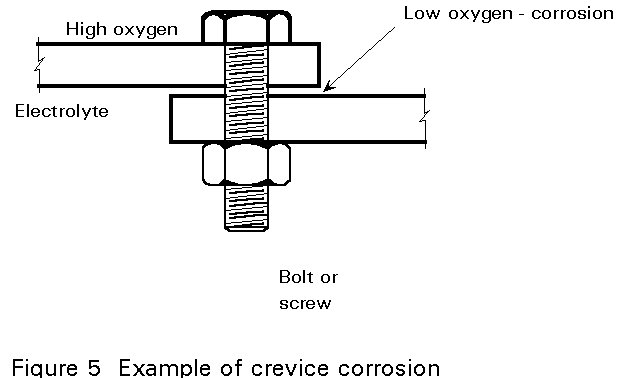
- Sixth, crevice corrosion occurs in the low oxygen concentration areas of a corrosion cell (see Figure 5).
- Seventh, even cold formed steel has anodic and cathodic areas allowing electro-chemical corrosion to occur (see Figure 6).